
Ontosight® – Biweekly NewsletterJune 17th – June 30th, 2024 –Read More

Ontosight® – Biweekly NewsletterJune 17th – June 30th, 2024 –Read More
May 24
Apr 24

Innoplexus wins Horizon Interactive Gold Award for Curia App
Read MoreThe success rate of clinical trials carries significant weight in the business of pharma. In addition to dedicating considerable time and effort, the industry spends billions on R&D every year. Clinical development requires optimal decision-making and multiple insights on various parameters, including identifying trial sites, recruiting patients, coordinating logistics, and overseeing research. Adding pressure to the situation is the fact that clinical trials fail about 9 times out of 10.1 The surprisingly low success rate is the result of such factors as failure to meet the enrollment targets up to 86% of the time, low efficacy results, poor endpoint statistics, and underpowered samples.2,3 Moreover, the dropout rate in phase III trials can be greater than 30%.4 Trials often run over budget or are plagued by research failure, clinical site fatigue, unskilled investigators, and high competition.
Only one in five candidates makes it from phase I trials to FDA approval, and nearly half of the compounds are abandoned before phase III trials for lack of efficacy. Of 5,000 to 10,000 chemical compounds put to preclinical testing, just 10 qualify for clinical stage.5 According to Policy & Medicine, this amounts to $2.6 billion spent on drug development, including large spends on failed compounds.1 Researchers at Johns Hopkins Bloomberg School of Public Health estimated a loss of approximately $6 million at phase I or II and around $77 million at phase III.6 Not to be ignored is that fact that the timeline for one approved drug to reach the market is 10-12 years from pipeline to commercialization.1 In fact, for drugs that target complex diseases, the approval rate is less than 0.1%.7 For instance, the failure rate for drugs developed between 2002 and 2012 targeting Alzheimer’s disease was 99.6%. Only 0.4% made it to phase III trials, and each of these had a 25%-50% probability of being approved.8
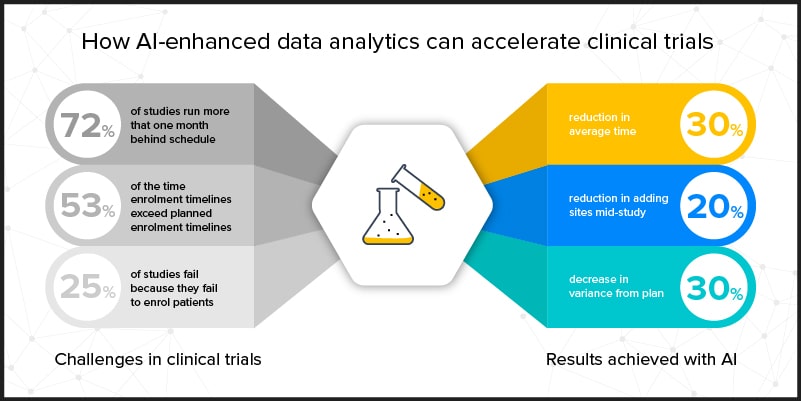
But there is hope. Leveraging the power of AI technology is the key to radically changing the biopharmaceutical industry with regard to the problem of length, cost, and success of clinical trials. Because AI can generate information that is fast, accurate, and efficient, it can deliver real-time, relevant insights that enable quick and accurate decision-making. The future success of the biopharmaceutical industry rests on its adoption of AI as a means to improve outcomes and costs of clinical trials.
All of the challenges in drug discovery and development discussed above can be addressed with AI. Leveraging fast-growing technologies such as neural network and deep learning to provide innovative solutions will accelerate clinical development. Because AI has the power to draw information from multiple life sciences data sources, it can be used to predict the outcome of clinical trials with great accuracy. In addition to searching through life sciences data, AI can also search on key opinion leaders involved, geographic availability of patients, endpoints used, and other factors that affect the success of clinical trials.
The ability of AI to greatly improve lead and target identification for drug development and drug repurposing will result in better optimization of clinical trials. The amount of life sciences data is a treasure trove of connections between biological entities, undiscovered connections, and hidden insights. To enable insights, leveraging technologies such as computer vision, network analysis, entity normalization are required. Faster and more comprehensive data analysis will lead to faster discoveries and successful trials.
The data in life sciences is growing at an extraordinary pace, and every new finding could be important for decision-making and drug development. In 2020, medical data is expected to double every 73 days.9 Researchers, most often PhDs, who should be focusing on decision-making for developing new therapies, spend 80% of their time on lower cognitive tasks such as collecting, curating, and cleaning data. A researcher spends about 2.5 hrs daily searching for relevant papers and another hour annotating and driving insights from it. With a new immunology paper published every half hour, it is impossible but important to generate relevant and accurate insights on time, as the ever-changing landscape of the industry needs quick results.
AI, on the other hand, generates key insights faster and from a larger bucket of data, leaving more time for scientists to focus on higher cognitive tasks. These insights can be used for identifying biological connections, in silico testing, drug repurposing, stratifying patients, finding clinical sites, identifying unmet needs, tracking share of voice, discovering thought leaders, predicting clinical trial results, staying abreast of the ever-changing regulatory landscape in pharma, and more. The effort required for data analytics and decision-making is greatly reduced and the time it takes to see results is accelerated with the use of AI.
Innoplexus leveraged its proprietary technologies to design the Clinical Trial Comparator Dashboard to identify and compare past and present, successful and failed trials, the Site Optimization & Enrollment Dashboard to enable informed selection of trial sites based on a multitude of parameters, and OntosightⓇ Influence, a KOL discovery, network analysis, and management tool. Moreover, a Clinical Trial Prediction engine was built to predict the success of trials to help reduce costs, efforts, and time wasted on failed trials.
Most clinical trials fail due to suboptimal site selection. It is important, therefore, to analyze the potential performance of clinical trial sites to determine and thereby mitigate the factors that are responsible for high costs and delays. For example, to generate insights and identify sites for a potential DLBCL therapy, Innoplexus facilitated the identification and prioritization of trial sites based on various parameters, including the presence of key opinion leaders, past involvement with a similar segmentation, competitor trials, geography, and patient populations. With customized machine learning algorithms, Innoplexus enabled benchmarking of the best sites and estimated their performance.
Key opinion leaders (KOLs) can be classified into asset class categories such as those with clinical trial experience and those who have authored published papers.. Also, distinguishing between top and emerging KOLs using network mapping can help choose the right ones for the trial. Innoplexus enables pharma companies to identify potential KOL partners by assessing, segmenting, and prioritizing leaders. These can be thought leaders in the medical and life sciences domain, pharma industry experts, presidents of medical foundations, and researchers. By facilitating access to untapped KOL networks in regards to the therapeutic area and disease subtypes, pharma companies can improve collaboration and commercialization.
With the use of AI, key insights generated about a given indication can enable scaled models and reduce time to estimate clinical trial costs. Innoplexus takes into account a number of factors, such as competitors’ trial size, duration, geographic site distribution, phase, sample size, indication, and principal investigators, and offers custom scoring for each. AI-based technologies, such as network analysis, help empower coordinators to identify the sites that are most likely to have underutilized KOLs and untapped patient populations.
AI-based data analytics can transform the face of clinical trials by offering previously unknown information and generating insights that could bring substantial potential to them. This will help reduce timelines and costs with informed decision-making.
References: 1. Sullivan T. A tough road: cost to develop one new drug is $2.6 billion; approval rate for drugs entering market is less than 12%. Policy & Medicine.
https://www.policymed.com/2014/12/a-tough-road-cost-to-develop-one-new-drug-is-26-billion-approval-rate-for-drugs-entering-clinical-de.html. Updated March 21, 2019. Accessed April 24, 2019. 2. Food and Drug Administration (FDA). Documents for patient engagement in medical device clinical trials meeting. FDA Executive Summary, Prepared for the October 11-12, 2017 Patient Engagement Advisory Committee. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PatientEngagementAdvisoryCommittee/UCM579153.pdf. Accessed April 24, 2019. 3. Huang GD, Bull J, Johnston McKee K, Mahon E, Harper B, Roberts JN; CTTI Recruitment Project Team. Clinical trials recruitment planning: A proposed framework from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2018;66:74-79. 4. Alexander W. The uphill path to successful clinical trials: keeping patients enrolled. PT. 2013;38(4):225-227. 5. Torjesen I. Drug development: the journey of a medicine from lab to shelf. Pharm J. https://www.pharmaceutical-journal.com/publications/tomorrows-pharmacist/drug-development-the-journey-of-a-medicine-from-lab-to-shelf/20068196.article. Published May 12, 2015. Accessed April 24, 2019. 6. Cost of Clinical Trials for New Drug FDA Approval Are Fraction of Total Tab. Johns Hopkins Bloomberg School of Public Health website. https://www.jhsph.edu/news/news-releases/2018/cost-of-clinical-trials-for-new-drug-FDA-approval-are-fraction-of-total-tab.htm. Published September 24, 2018. Accessed April 24, 2019. 7. Rai A, Shinde P, Jalan S. Network spectra for drug-target identification in complex diseases: new guns against old foes. Appl Netw Sci. 2018;3(1):51. 8. Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37. 9. Densen P. Challenges and opportunities facing medical education. Trans Am Clin Climatol Assoc. 2011;122:48-58.
The cost of developing a new drug roughly doubles every nine years (inflation-adjusted) aka Eroom’s law. As the volume of data…
There was a time when science depended on manual efforts by scientists and researchers. Then, came an avalanche of data…
Collaboration with key opinion leaders and influencers becomes crucial at various stages of the drug development chain. When a pharmaceutical…
Data are not the new gold – but the ability to put them together in a relevant and analyzable way…
Artificial intelligence, or AI, is gaining more attention in the pharma space these days. At one time evoking images from…
Artificial intelligence (AI) is transforming the pharmaceutical industry with extraordinary innovations that are automating processes at every stage of drug…
There is a lot of buzz these days about how artificial intelligence (AI) is going to disrupt the pharmaceutical industry….
Drug discovery plays a key role in the pharma and biotech industries. Discovering unmet needs, pinpointing the target, identifying the…
The pharmaceutical industry spends billions on R&D each year. Clinical trials require tremendous amounts of effort, from identifying sites and…
Training algorithms to identify and extract Life Sciences-specific data The English dictionary is full of words and definitions that can be…
The early 1970s introduced the world to the idea of computer vision, a promising technology automating tasks that would otherwise…
Summary: AI could potentially speed drug discovery and save time in rejecting treatments that are unlikely to yield worthwhile resultsAI has…